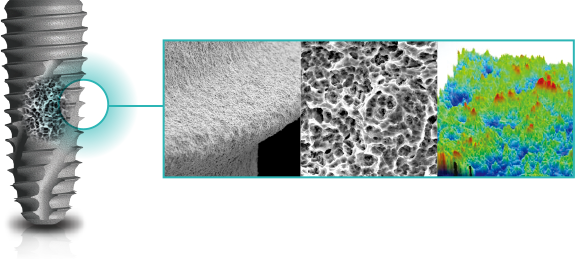
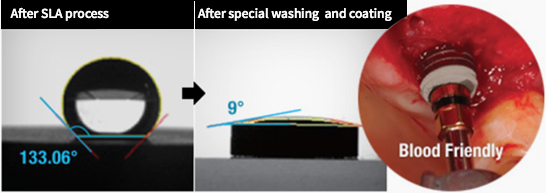
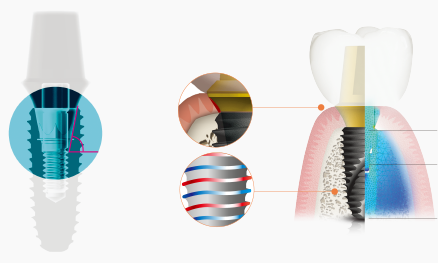
COWELLMEDI INNO SLA-SH® Dental Implant is
the symbolic product of COWELLMEDI, which succeeded
in localizing for the first time in South Korea. It is the
product with an internationally proven quality and safety
developed with know-how accumulated for 20 years of
research. And it increases the completeness of the
implant procedure with the state-of-art biocompatible
surface treatment (SLA-SH®).





 USFDA Certification
USFDA Certification European Quality
European Quality ISO 13485 Certification
ISO 13485 Certification Ministry of Food and
Ministry of Food and CNC Machining
CNC Machining Surface Treatment
Surface Treatment Inspection
Inspection Cleansing
Cleansing Packing/Sterilization
Packing/Sterilization Shipping Warehouse
Shipping Warehouse