COWELL NEWS - JANUARY VOL.1
Cowellmedi / 2021-01-08
|
COWELL NEWS
JANUARY VOL.1
8 JANUARY 2021
CE Mark approved Titan-X and DIA-DERM® M
Introducing the new Gold Standard in Xenograft, Titan-X and
Biodegradable Atelocollagen Membrane, DIA-DERM® M!
Titan-X
Human Biopsy Result
Osteoconductive nature of the TITAN-X surface was evaluated by biopsy specimens. Consistent New bone formations were noted in several difference clinical cases: Reliability of graft success, early bone formation, and observation of osteocyte lacunae.
Born to be OSTEOCONDUCTIVE
Multiporosity Structure
The TITAN-X is made of 100% cancellous bone without any cortical portion.Innovative pulverizing technique allows multiparous structure, maximizing blood vessel ingrowth.
Osteoconductive Surface
Low temperature processing technique allows ideal, natural surface topograph,the same as human bone, stimulating osteoblast activity.Vitrification phenomenon caused by high temperature process has been completelycontrolled.
Calcium Phosphate Crystal
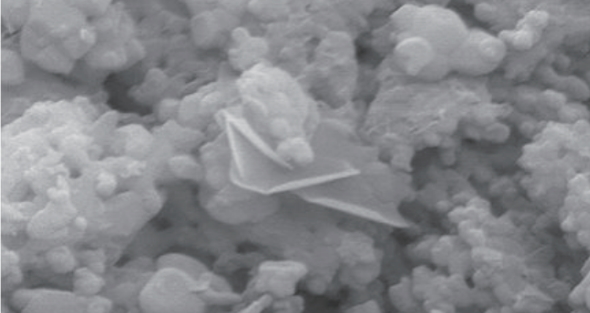
Pre HA structure, octacalcium phosphate crystal is found on the surface of the TITAN-X, resulting in fast bone formation.
Pore Dimension
Microscopic Comparison
TITAN-X
Others
Certification
Specification
DIADERM® M
Product Features
Resistance against enzymatic degradation
DIADERM® M has resistance to enzymatic degradation compared to products from other manufacturers.
Certification
Specification
Connect with us on social media
©2020 Cowellmedi Co., Ltd. All rights reserved
COWELLMEDI Int'l Project Development
Floor 6, Blue Fin Tower, 42, Seochojungang-ro, Seocho-gu, Seoul, 06643, Republic of Korea
Tel. +82 2 3453 5085 Telefax. +82 2 3453 5086
Email. isp@cowellmedi.com
www.cowellmedi.com |